EXHIBIT 99.1
Published on January 12, 2026
Exhibit 99.1
Exhibit 99.1

1 Corporate Presentation Q1 2026

2 Legal Disclaimer This presentation of Pelthos Therapeutics Inc. (“we”, “us”, “our” or the “Company”) contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act and other securities laws. Words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict, “project,” “seek,” “should,” “target,” “will,” “would” or similar expressions and the negatives of those term are intended to identify forward-looking statements. Forward-looking statements reflect management’s current expectations, are based on judgments and assumptions, are inherently uncertain and are subject to risks, uncertainties and other factors, which could cause the Company’s actual results, performance or achievements to differ materially from expected future results, performance or achievements expressed or implied in those forward-looking statements. Examples of these forward-looking statements and the related risks, uncertainties and other factors include, but are not limited to, the following: the success of the launch for Zelsuvmi, timing, progress and results of any preclinical and clinical trials, its estimates regarding the potential market opportunity for Zelsuvmi, its ability to develop its pipeline, its ability to protect its intellectual property and enforce its intellectual property rights, and its ability to execute its development strategy and sustain its competitive position. Actual future results and trends may differ materially depending on a variety of factors, including, but not limited to, the Company’s limited operating history, the Company’s ability to establish its market development capabilities to commercialize its products and generate any revenue, and the Company’s ability to maintain regulatory approval of Zelsuvmi. Forward-looking statements are provided to allow potential investors the opportunity to understand management’s beliefs and opinions in respect of the future so that they may use such beliefs and opinions as one factor in evaluating an investment. These statements are not guarantees of future performance and undue reliance should not be placed on them. Any forward-looking statement in this presentation, in any related presentation supplement and in any related free writing presentation reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our business, results of operations, industry and future growth. You should read this presentation with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.

3 3 • Large addressable market with $2,008.50 wholesale acquisition cost (”WAC”) • Launched July 2025 • Modest acquisition cost, unencumbered future revenue stream • Expected launch in late 2026 • 6–12 million U.S. cases annually • Expected launch during the first half of 2027 Investment Highlight ? Commercial biopharmaceutical company focused on growing, differentiated cutaneous infections product portfolio ? Highly synergistic Xepi and Xeglyze product acquisitions leverage Zelsuvmi’s current commercial and market access team and infrastructure ? Strong potential revenue streams with very attractive gross to nets ? Disciplined, accretive, cost-efficient product acquisition model and experienced management team to manage execution Product Portfolio
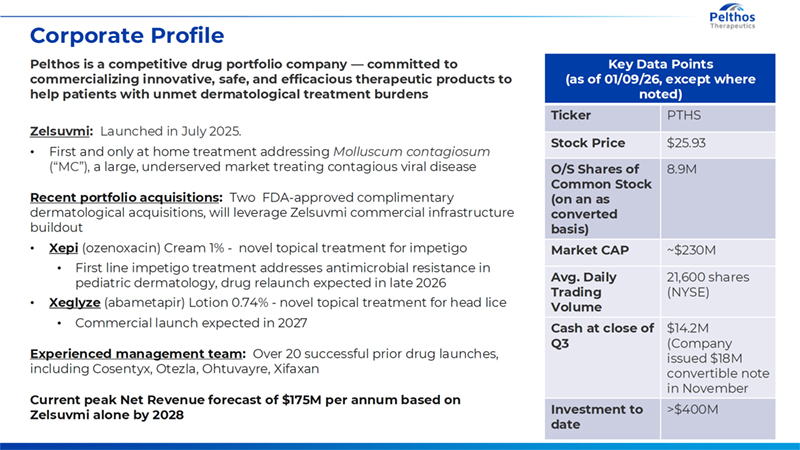
4 Corporate Profile Key Data Points (as of 01/09/26, except where noted) Ticker PTHS Stock Price $25.93 O/S Shares of Common Stock (on an as converted basis) 8.9M Market CAP ~$230M Avg. Daily Trading Volume 21,600 shares (NYSE) Cash at close of Q3 $14.2M (Company issued $18M convertible note in November Investment to date >$400M Pelthos is a competitive drug portfolio company — committed to commercializing innovative, safe, and efficacious therapeutic products to help patients with unmet dermatological treatment burdens Zelsuvmi: Launched in July 2025. • First and only at home treatment addressing Molluscum contagiosum (“MC”), a large, underserved market treating contagious viral disease Recent portfolio acquisitions: Two FDA-approved complimentary dermatological acquisitions, will leverage Zelsuvmi commercial infrastructure buildout • Xepi (ozenoxacin) Cream 1% - novel topical treatment for impetigo • First line impetigo treatment addresses antimicrobial resistance in pediatric dermatology, drug relaunch expected in late 2026 • Xeglyze (abametapir) Lotion 0.74% - novel topical treatment for head lice • Commercial launch expected in 2027 Experienced management team: Over 20 successful prior drug launches, including Cosentyx, Otezla, Ohtuvayre, Xifaxan Current peak Net Revenue forecast of $175M per annum based on Zelsuvmi alone by 2028

5 Management Team Frank Knuettel | Chief Financial Officer Sai Rangarao | Chief Commercial Officer Scott Plesha | Chief Executive Officer • 30 years of management experience in growing early-stage companies • Raised more than $400 million viaventure, public equity and debt offerings and managed more than 15 mergersand acquisition transactions along with large-scale licensing transactions with fortune 50companies • Holds numerous board positions, at both public andprivate companies, including Etheros Pharmaceuticals • Earned an MBA from The Wharton School and a BA from Tufts University • >30 years of experience in the pharmaceutical industry, including two decades building andleading specialty pharma commercial efforts • President and Chief Commercial Officer at BDSI until it was acquired by Collegium Pharmaceutical in 2022 • Grew BDSI sales from $5 million to $160 million • Previously served as Senior Vice President of Gastrointestinal Sales at SalixPharmaceuticals. During fifteen-year tenure at Salix, led nationwide salesforce that grew product sales to more than $1.5 billion annually • Earned a BA inPre-Medicine and Pre-Medical Studies at DePauw University and pursued graduatestudies in Dentistry at Indiana University Dental School • >18 years of experience leading, launching, and marketing pharmaceutical products • VP of Marketing & Head of Neurology Sales at Collegium Pharmaceutical • VP of Marketing & Commercial Operations at BDSI, until it was acquired by Collegium in 2022 • Head of US Dermatology Marketing for Otezla at Celgene leading to acquisition by Amgen for $13 Billion • Member of the commercial and marketing organization at Novartis Pharmaceuticals that launched COSENTYX®in the U.S • Earned an MS in Bioscience Regulatory Affairs from The Johns Hopkins University, an MBA and MS from the New Jersey Institute of Technology, and a BS in Computer Science from Indiana University of Pennsylvania

6 Board of Directors Peter Greenleaf, Chairman Richard Baxter Ezra Friedberg Todd Davis Richard Malamut , MD Matt Pauls Scott Plesha Andrew Einhorn

molluscum & Zelsuvmi Overview
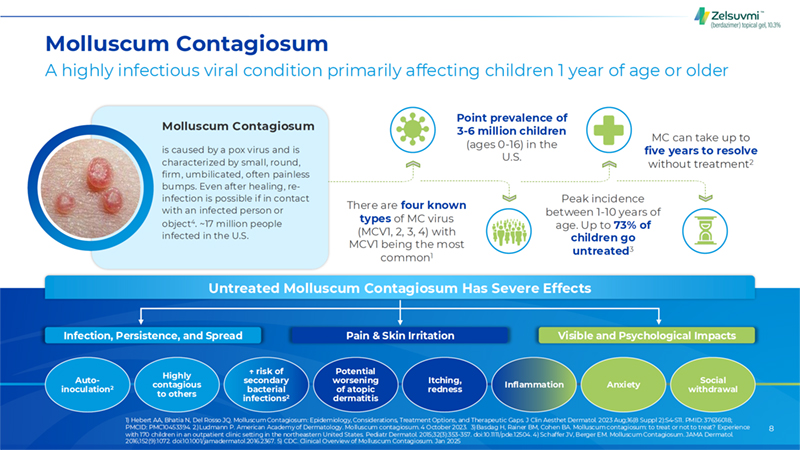
8 Molluscum Contagiosum A highly infectious viral condition primarily affecting children 1 year of age or older There are four known types of MC virus (MCV1, 2, 3, 4) with MCV1 being the most common1 Molluscum Contagiosum is caused by a pox virus and is characterized by small, round, firm, umbilicated, often painless bumps. Even after healing, re-infection is possible if in contact with an infected person or object4. ~17 million people infected in the U.S. MC can take up to five years to resolve without treatment2 Peak incidence between 1-10 years of age. Up to 73% of children go untreated3 Point prevalence of 3-6 million children (ages 0-16) in the U.S. Infection, Persistence, and Spread Visible and Psychological Impacts Pain & Skin Irritation Auto- inoculation2 Highly contagious to others ? risk of secondary bacterial infections2 Potential worsening of atopic dermatitis Itching, redness Inflammation Anxiety Social withdrawal Untreated Molluscum Contagiosum Has Severe Effects 1) Hebert AA, Bhatia N, Del Rosso JQ. Molluscum Contagiosum: Epidemiology, Considerations, Treatment Options, and Therapeutic Gaps. J Clin Aesthet Dermatol. 2023 Aug;16(8 Suppl 2):S4-S11. PMID: 37636018; PMCID: PMC10453394. 2.)Ludmann P. American Academy of Dermatology. Molluscum contagiosum. 4 October 2023. 3) Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? Experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32(3):353-357. doi:10.1111/pde.12504. 4) Schaffer JV, Berger EM. Molluscum Contagiosum. JAMA Dermatol. 2016;152(9):1072. doi:10.1001/jamadermatol.2016.2367. 5) CDC. Clinical Overview of Molluscum Contagiosum. Jan 2025
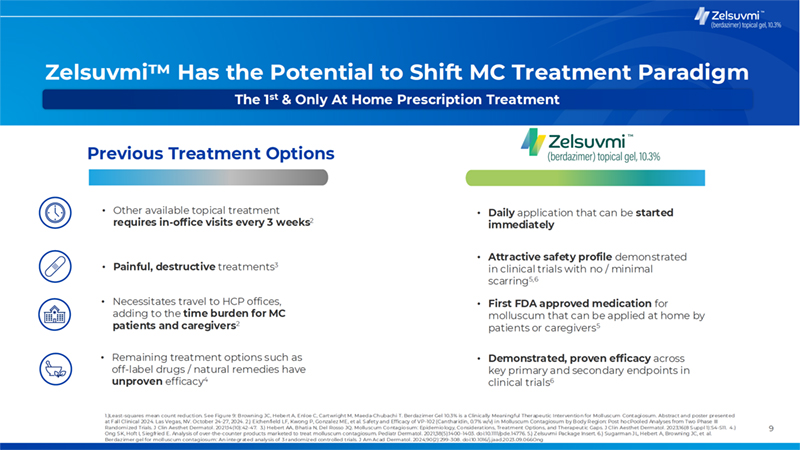
9 Zelsuvmi Has the Potential to Shift MC Treatment Paradigm Previous Treatment Options • Painful, destructive treatments3 • Attractive safety profile demonstrated in clinical trials with no / minimal scarring5,6 • Remaining treatment options such as off-label drugs / natural remedies have unproven efficacy4 • Demonstrated, proven efficacy across key primary and secondary endpoints in clinical trials6 • Necessitates travel to HCP offices, adding to the time burden for MC patients and caregivers2 • First FDA approved medication for molluscum that can be applied at home by patients or caregivers5 • Other available topical treatment requires in-office visits every 3 weeks2 • Daily application that can be started immediately 1.)Least-squares mean count reduction. See Figure 9: Browning JC, Hebert A, Enloe C, Cartwright M, Maeda-Chubachi T. Berdazimer Gel 10.3% is a Clinically Meaningful Therapeutic Intervention for Molluscum Contagiosum. Abstract and poster presented at Fal l Cl inical 2024. Las Vegas, NV. October 24-27, 2024. 2.) Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and Efficacy of VP-102 (Cantharidin, 0.7% w/v) in Molluscum Contagiosum by Body Region: Post hoc Pooled Analyses from Two Phase III Randomized Trials. J Clin Aesthet Dermatol. 2021;14(10):42-47. 3.) Hebert AA, Bhatia N, Del Rosso JQ. Molluscum Contagiosum: Epidemiology, Considerations, Treatment Options, and Therapeutic Gaps. J Cl in Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11. 4.) Ong SK, Hoft I, Siegfried E. Analysis of over-the-counter products marketed to treat molluscum contagiosum. Pediatr Dermatol . 2021;38(5):1400-1403. doi:10.1111/pde.14776. 5.) Zelsuvmi Package Insert. 6.) Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: An integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90(2):299-308. doi:10.1016/j.jaad.2023.09.066Ong The 1st & Only At Home Prescription Treatment
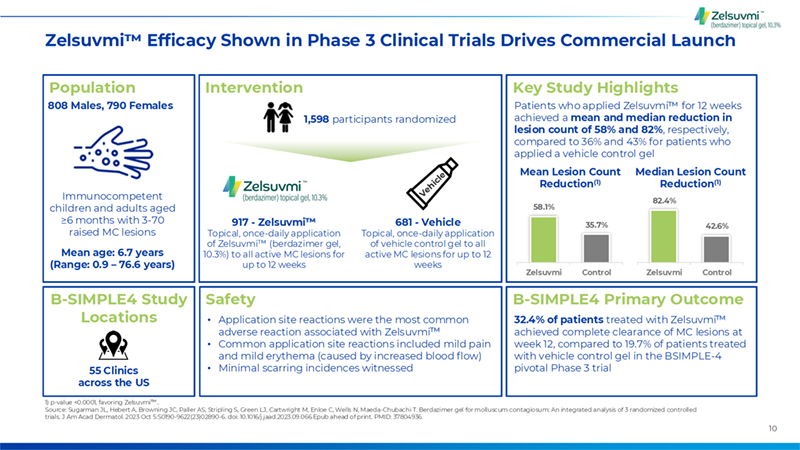
10 Intervention Population Key Study Highlights B-SIMPLE4 Primary Outcome B-SIMPLE4 Study Locations 1) p-value <0.0001, favoring Zelsuvmi .. Source: Sugarman JL, Hebert A, Browning JC, Paller AS, Stripling S, Green LJ, Cartwright M, Enloe C, Wells N, Maeda-Chubachi T. Berdazimer gel for molluscum contagiosum: An integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2023 Oct 5:S0190-9622(23)02890-6. doi: 10.1016/j.jaad.2023.09.066.Epub ahead of print. PMID: 37804936. Zelsuvmi Efficacy Shown in Phase 3 Clinical Trials Drives Commercial Launch 808 Males, 790 Females Immunocompetent children and adults aged =6 months with 3-70 raised MC lesions Mean age: 6.7 years (Range: 0.9 – 76.6 years) 1,598 participants randomized 917 - Zelsuvmi Topical, once-daily application of Zelsuvmi (berdazimer gel, 10.3%) to all active MC lesions for up to 12 weeks 681 - Vehicle Topical, once-daily application of vehicle control gel to all active MC lesions for up to 12 weeks Patients who applied Zelsuvmi for 12 weeks achieved a mean and median reduction in lesion count of 58% and 82%, respectively, compared to 36% and 43% for patients who applied a vehicle control gel Mean Lesion Count Reduction(1) 58.1% 35.7% Zelsuvmi Control 55 Clinics across the US 32.4% of patients treated with Zelsuvmi achieved complete clearance of MC lesions at week 12, compared to 19.7% of patients treated with vehicle control gel in the BSIMPLE-4 pivotal Phase 3 trial Median Lesion Count Reduction(1) 82.4% 42.6% Zelsuvmi Control Safety • Application site reactions were the most common adverse reaction associated with Zelsuvmi • Common application site reactions included mild pain and mild erythema (caused by increased blood flow) • Minimal scarring incidences witnessed
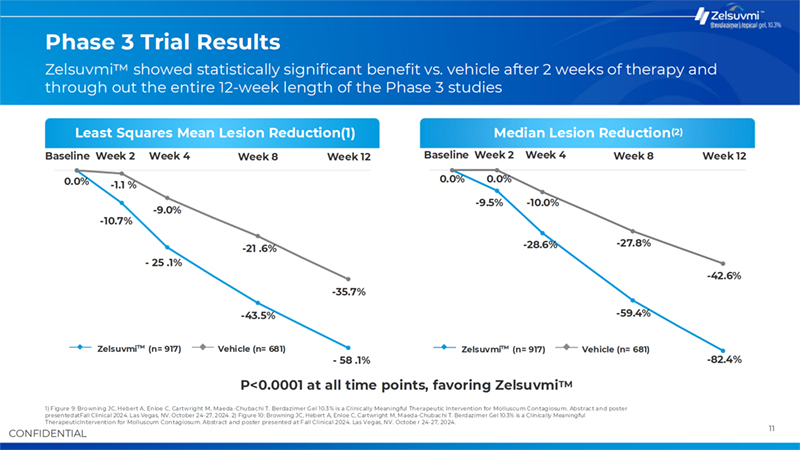
11 CONFIDENTIAL Baseline Week 2 Week 4 0.0% -1.1 % -43.5% Week 8 -35.7% Week 12 -59.4% -82.4% -42.6% -10.7% - 25 .1% -9.0% -21 .6% - 58 .1% Baseline Week 2 Week 4 0.0% 0.0% -9.5% -10.0% -28.6% -27.8% Week 8 Week 12 Phase 3 Trial Results 1) Figure 9: Browning JC, Hebert A, Enloe C, Cartwright M, Maeda-Chubachi T. Berdazimer Gel 10.3% is a Clinically Meaningful Therapeutic Intervention for Molluscum Contagiosum. Abstract and poster presentedatFall Clinical 2024. Las Vegas, NV. October 24-27, 2024. 2) Figure 10: Browning JC, Hebert A, Enloe C, Cartwright M, Maeda-Chubachi T. Berdazimer Gel 10.3% is a Clinically Meaningful TherapeuticIntervention for Molluscum Contagiosum. Abstract and poster presented at Fall Clinical 2024. Las Vegas, NV. Octobe r 24-27, 2024. Least Squares Mean Lesion Reduction(1) P<0.0001 at all time points, favoring Zelsuvmi Median Lesion Reduction TM (2) Zelsuvmi (n= 917) Vehicle (n= 681) Zelsuvmi showed statistically significant benefit vs. vehicle after 2 weeks of therapy and through out the entire 12-week length of the Phase 3 studies Zelsuvmi (n= 917) Vehicle (n= 681)

Zelsuvmi Commercial Overview
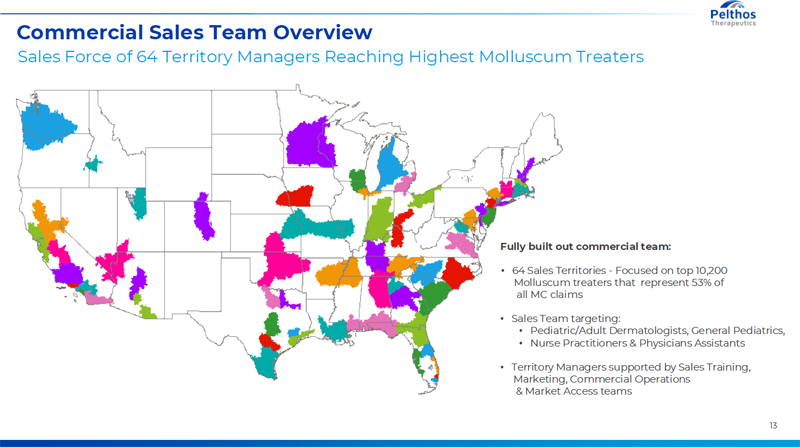
13 Commercial Sales Team Overview Sales Force of 64 Territory Managers Reaching Highest Molluscum Treaters Fully built out commercial team: ? 64 Sales Territories - Focused on top 10,200 Molluscum treaters that represent 53% of all MC claims ? Sales Team targeting: ? Pediatric/Adult Dermatologists, General Pediatrics, ? Nurse Practitioners & Physicians Assistants ? Territory Managers supported by Sales Training, Marketing, Commercial Operations & Market Access teams
14 2026 Zelsuvmi Tactical Overview KOL Education Digital Marketing National & Regional Conference Presence CRM Platform: Education & Communication YouTube Promotional Video Live & Virtual Educational Speaker Development New Patient Testimonials and Advertisements ZELSUVMI GO Patient Support Program
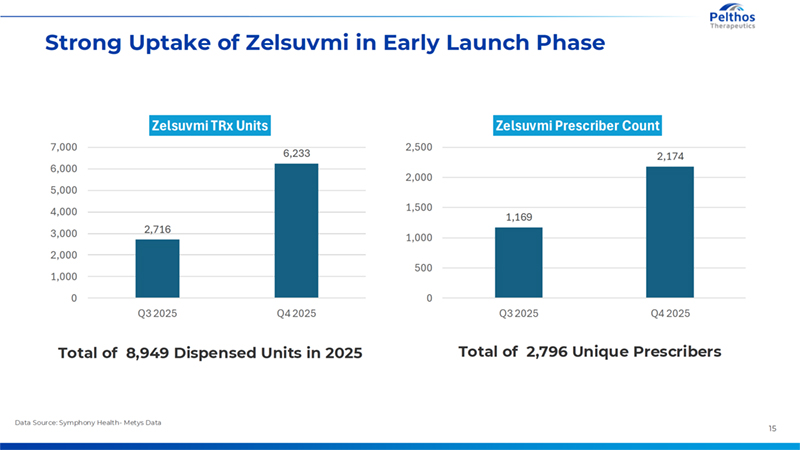
15 Strong Uptake of Zelsuvmi in Early Launch Phase Data Source: Symphony Health- Metys Data Total of 2,796 Unique Prescribers Total of 8,949 Dispensed Units in 2025 2,716 6,233 0 1,000 2,000 3,000 4,000 5,000 6,000 7,000 Q3 2025 Q4 2025 Zelsuvmi TRx Units 1,169 2,174 0 500 1,000 1,500 2,000 2,500 Q3 2025 Q4 2025 Zelsuvmi Prescriber Count
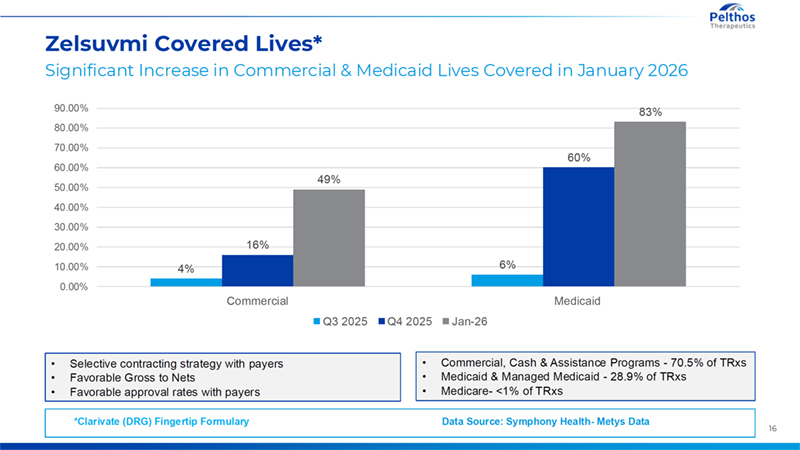
16 Zelsuvmi Covered Lives* Significant Increase in Commercial & Medicaid Lives Covered in January 2026 *Clarivate (DRG) Fingertip Formulary Data Source: Symphony Health- Metys Data 4% 6% 16% 60% 49% 83% 0.00% 10.00% 20.00% 30.00% 40.00% 50.00% 60.00% 70.00% 80.00% 90.00% Commercial Medicaid Q3 2025 Q4 2025 Jan-26 • Selective contracting strategy with payers • Favorable Gross to Nets • Favorable approval rates with payers • Commercial, Cash & Assistance Programs - 70.5% of TRxs • Medicaid & Managed Medicaid - 28.9% of TRxs • Medicare- <1% of TRxs

Xepi: New Product Acquisition
Xepi(ozenoxacin) Cream for the treatment of Impetigo Sources: CDC Website, Xepi Pack Insert, FDA.gov Ozenoxacincream 1% developed as first line treatment in patients aged 2 months and older 15 clinical studies in Phase 1 & 2 conducted Two Pivotal Phase 3 studies conducted in both adult & pediatric patients with impetigo 2 months old and up Ozenoxacindemonstrated superior clinical and bacteriological outcomes vs. vehicle control XepiClinical Story #1 bacterial infection seen in pediatrician offices, represents 1-2% of all visits to Pediatricians in the US, with 135Mchildren suffering worldwide Impetigo is a highly contagious bacterial skin infection, most often caused by Staphylococcus aureus and/or Group A Streptococcus (Streptococcus pyogenes) Mupirocin resistance is growing significantly in the US Impetigo Facts1 Strong synergy with existing commercial infrastructure for Zelsuvmi Significant overlap between Xepi& Zelsuvmi HCP call points Promotional alignment across Sales, Marketing & Commercial Operations Anticipated Commercial Launch: Late 2026 Pelthos Opportunity Acquired from BioFrontera in October 2025 FDA Approved in 2017 Exclusivity until 2032 1https://www.cdc.gov/group-a-strep/about/impetigo.html 18

Xeglyze: New Product Acquisition
Xeglyze (abametapir) Lotion for the Treatment of Head Lice Sources: CDC Website, XepiPack Insert, FDA.gov Abametapirlotion 0.74% developed as first line treatment in patients aged 6 months of age and older Phase 2b study completed in 2014 demonstrated 100% ovicidal efficacy Two Pivotal Phase 3 studies demonstrated that a single, ten-minute application of Xeglyze® results in a statistically significant increase in the proportion of subjects who are cleared of lice versus vehicle. Xeglyze Clinical Story 100m+infestations globally, with 6-12m cases in the US, each year with substantial social cost Increasing resistance to current products containing pyrethrin, permethrin & malathion Existing products are only effective against lice and not eggs, and most require repeat treatments to break life cycle of infestation, leading to poor compliance and reduced efficacy Head Lice Facts1 Strong synergy with existing commercial infrastructure for Zelsuvmi and Xepi Significant overlap between Xeglyze& Zelsuvmi HCP call points Promotional alignment across Sales, Marketing & Commercial Operations Anticipated Commercial Launch: First half 2027 PelthosOpportunity Acquired from Hatchtech in December 2025 FDA Approved in 2020 Exclusivity until 2034 1https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208945lbl.pdf 20

NitricilTM Platform & NaV1.7 Pipeline Overview
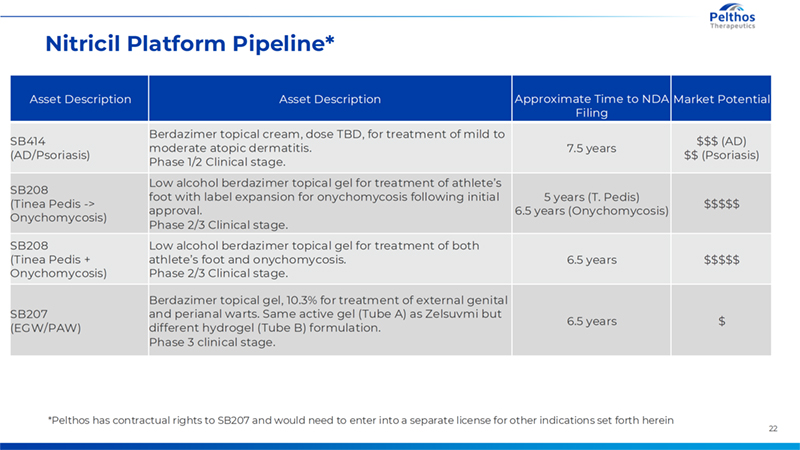
22 Nitricil Platform Pipeline* Asset Description Asset Description Approximate Time to NDA Filing Market Potential SB414 (AD/Psoriasis) Berdazimer topical cream, dose TBD, for treatment of mild to moderate atopic dermatitis. Phase 1/2 Clinical stage. 7.5 years $$$ (AD) $$ (Psoriasis) SB208 (Tinea Pedis -> Onychomycosis) Low alcohol berdazimer topical gel for treatment of athlete’s foot with label expansion for onychomycosis following initial approval. Phase 2/3 Clinical stage. 5 years (T. Pedis) 6.5 years (Onychomycosis) $$$$$ SB208 (Tinea Pedis + Onychomycosis) Low alcohol berdazimer topical gel for treatment of both athlete’s foot and onychomycosis. Phase 2/3 Clinical stage. 6.5 years $$$$$ SB207 (EGW/PAW) Berdazimer topical gel, 10.3% for treatment of external genital and perianal warts. Same active gel (Tube A) as Zelsuvmi but different hydrogel (Tube B) formulation. Phase 3 clinical stage. 6.5 years $ *Pelthos has contractual rights to SB207 and would need to enter into a separate license for other indications set forth herein
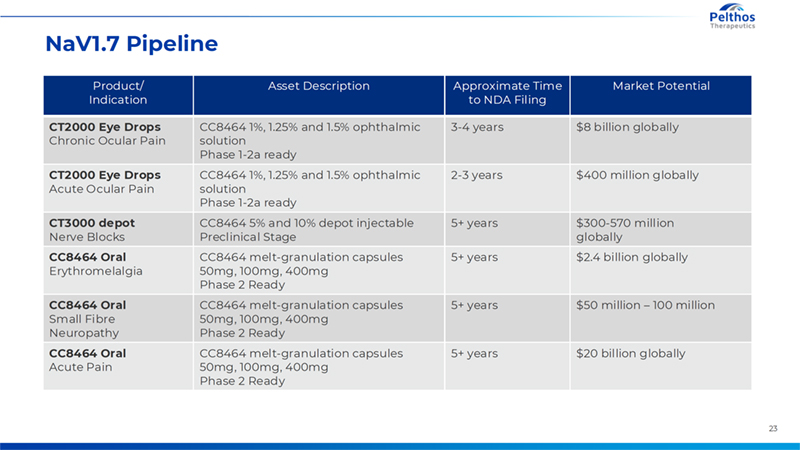
23 NaV1.7 Pipeline Product/ Indication Asset Description Approximate Time to NDA Filing Market Potential CT2000 Eye Drops Chronic Ocular Pain CC8464 1%, 1.25% and 1.5% ophthalmic solution Phase 1-2a ready 3-4 years $8 billion globally CT2000 Eye Drops Acute Ocular Pain CC8464 1%, 1.25% and 1.5% ophthalmic solution Phase 1-2a ready 2-3 years $400 million globally CT3000 depot Nerve Blocks CC8464 5% and 10% depot injectable Preclinical Stage 5+ years $300-570 million globally CC8464 Oral Erythromelalgia CC8464 melt-granulation capsules 50mg, 100mg, 400mg Phase 2 Ready 5+ years $2.4 billion globally CC8464 Oral Small Fibre Neuropathy CC8464 melt-granulation capsules 50mg, 100mg, 400mg Phase 2 Ready 5+ years $50 million – 100 million CC8464 Oral Acute Pain CC8464 melt-granulation capsules 50mg, 100mg, 400mg Phase 2 Ready 5+ years $20 billion globally
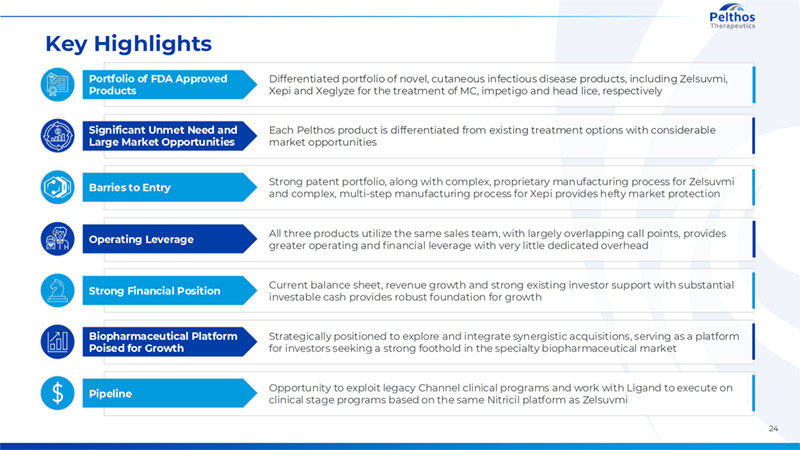
24 Operating Leverage All three products utilize the same sales team, with largely overlapping call points, provides greater operating and financial leverage with very little dedicated overhead Portfolio of FDA Approved Products Differentiated portfolio of novel, cutaneous infectious disease products, including Zelsuvmi, Xepi and Xeglyze for the treatment of MC, impetigo and head lice, respectively Barries to Entry Strong patent portfolio, along with complex, proprietary manufacturing process for Zelsuvmi and complex, multi-step manufacturing process for Xepi provides hefty market protection Significant Unmet Need and Large Market Opportunities Each Pelthos product is differentiated from existing treatment options with considerable market opportunities Strong Financial Position Current balance sheet, revenue growth and strong existing investor support with substantial investable cash provides robust foundation for growth Pipeline Opportunity to exploit legacy Channel clinical programs and work with Ligand to execute on clinical stage programs based on the same Nitricil platform as Zelsuvmi Biopharmaceutical Platform Poised for Growth Strategically positioned to explore and integrate synergistic acquisitions, serving as a platform for investors seeking a strong foothold in the specialty biopharmaceutical market Key Highlights

25 Thank You ContactsInvestor Inquiries:Mike MoyerLifeSci Advisors, LLCManaging Directormmoyer@lifesciadvisors.comMedia:KWM CommunicationsKellie Walsh / Rachel Kesslerpelthos@kwmcommunications.com(914) 315-6072